Getting your Trinity Audio player ready...
The Health Ministry reported Sunday that for the first time Since July 2, Israel is host to less than 10,000 active coronavirus cases.
Out of the 9,762 patients battling the virus, 392 are in serious condition, with 171 connected to ventilators.
The ministry also reported that the death toll from the disease also rose to 2,553.
With 329 new diagnoses out of 8,360 coronavirus tests by Sunday evening, Israel's infection rate stands at 4%.
Prime Minister Benjamin Netanyahu and Defense Minister Benny Gantz visited the hospital where the clinical trials for coronavirus vaccine began early Sunday, praising the first volunteers.
3 View gallery
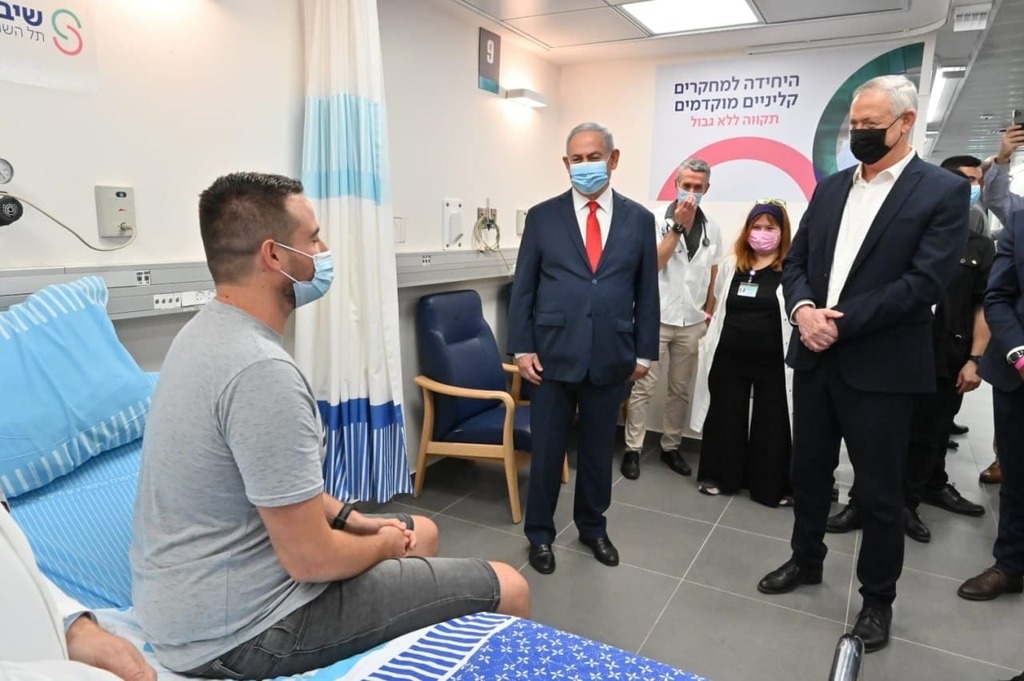

PM Netanyahu and Defense Minister Gantz meet with vaccine volunteer Segev Harel
(Photo: Defense Ministry)
Israel's Institute for Biological Research in Ness Ziona, tasked with developing the Israeli vaccine, announced last month it was to begin the clinical trials phase on November 1 with about one hundred Israelis aged 18-55 expected to participate by the time the trials are over. The first phase is taking at Sheba Medical Center in Tel HaShomer and Hadassah Medical Center in Jerusalem.
The first dose was administered to a 26-year-old volunteer, Segev Harel, from Kibbutz Sde Nehemia in northern Israel. He was greeted by both Netanyahu and Gantz at Sheba Medical Center.
Director General of the Israel Institute for Biological Research Shmuel Shapira said the third phase of the human trials will take place overseas, in a country with a high coronavirus infection rate.
Eighty volunteers will initially take part in the trial that will be expanded to 960 people in December. Should those trials succeed, a third stage with 30,000 volunteers is scheduled for April/May.
The institute, which is overseen by the Defense Ministry, began animal trials for its "BriLife" vaccine in March.
Shmuel Yitzhaki, head of the institute's biology division, said that if all goes well the vaccine could reach the general population by the end of next summer.
Reuters contributed to this article
First published: 19:53, 11.01.20



