Getting your Trinity Audio player ready...
Prime Minister Benjamin Netanyahu and Defense Minister Benny Gantz visited the hospital where the clinical trials for coronavirus vaccine began early Sunday, praising the first volunteers.
Israel's Institute for Biological Research in Ness Ziona, tasked with developing the Israeli vaccine, announced last month it was to begin the clinical trials phase on November 1 with about one hundred Israelis aged 18-55 expected to participate by the time the trials are over. The first phase is taking at Sheba Medical Center in Tel HaShomer and Hadassah Medical Center in Jerusalem.
3 View gallery


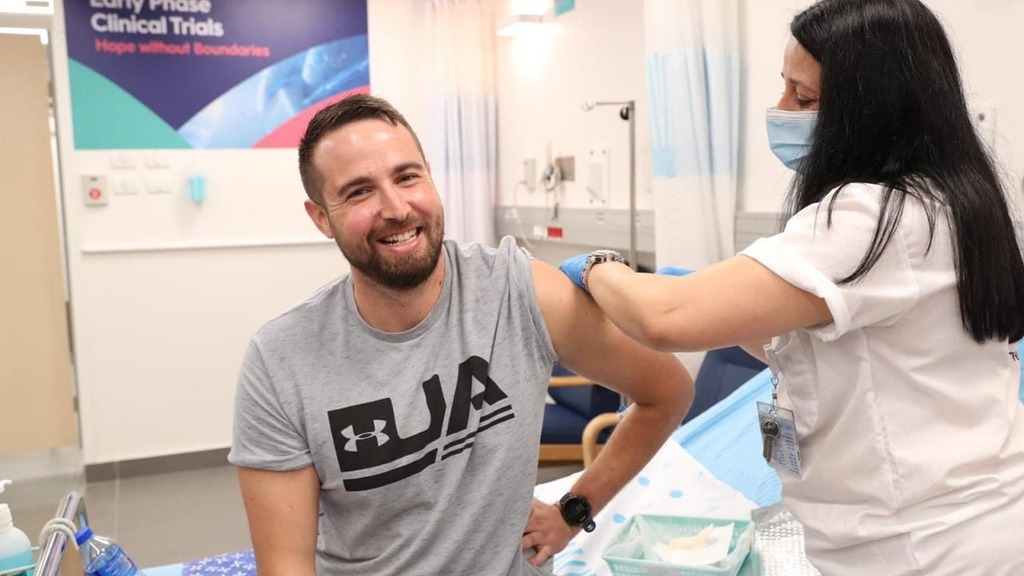
Netanyahu and Gantz meet with vaccine volunteer Segev Harel
(Photo: The Defense Ministry )
The first dose was administered to a 26-year-old volunteer, Segev Harel, from Kibbutz Sde Nehemia in northern Israel. He was greeted by both Netanyahu and Gantz at Sheba Medical Center.
"I see the light at the end of the tunnel now, this will be the real way out of this crisis," said the prime minister during the meeting.
"Israel was one of the first countries in the world to impose a lockdown. Now that we're exiting it, other countries are entering. Almost all of Europe is entering a closure of some sort," Netanyahu said, adding that Israel will help high street vendors to survive the sputtering of the economy.
"What is important is that one way or another, through domestic production or through deliveries of vaccines from abroad, we provide vaccines to all Israeli citizens."
Gantz said that Israelis must be "patient" while waiting for the vaccine and it will be a "long process".
"[The volunteer] told me, 'I came here to ask for financial help.' I replied to him that the prime minister is busy maintaining our security and we will take care of the economy when we'll draft the 2021 budget," Gantz said.
Director General of the Israel Institute for Biological Research Shmuel Shapira said the third phase of the human trials will take place overseas, in a country will high coronavirus infection rate.
3 View gallery


Director General of the Israel Institute for Biological Research Shmuel Shapira
(Photo: The Knesset Channel )
Eighty volunteers will initially take part in the trial that will be expanded to 960 people in December. Should those trials succeed a third stage with 30,000 volunteers is scheduled for April/May.
The institute, which is overseen by the Defense Ministry, began animal trials for its "BriLife" vaccine in March. Shmuel Yitzhaki, head of the institute's biology division, said that if all goes well the vaccine could reach the general population by the end of next summer.
Reuters contributed to this report