Getting your Trinity Audio player ready...
Despite most individuals linking sleep apnea to the elderly, children may suffer from it as well. This rare and life-threatening neurological condition is called Congenital Central Hypoventilation Syndrome (CCHS), also known as “Ondine's Curse."
More stories:
The nickname originates from a German myth, which formed the basis for "The Little Mermaid," according to which a mythological nymph named Ondine cursed her human husband after he cheated on her with another woman, causing him to lose the ability to breathe by himself.
The syndrome affects the normal function of the autonomic nervous system, and individuals suffering it immediately stop breathing when they fall asleep. CCHS patients are essentially under constant threat to their lives if they fall asleep without being connected to a medical ventilator.
According to statistical data, there one out of 120,000 babies in Israel is affected. It’s reasonable to assume that the prevalence of the disease is higher and includes undiagnosed cases of sudden infant death syndrome (SIDS).
A new Israeli study, conducted in collaboration between Ben-Gurion University and Tel Aviv University, the findings of which were published in the prestigious EMBO Journal, sheds light on the genetic mutations that cause this syndrome and what can be done to prevent the condition.
The mutation behind ‘Ondine’s Curse’
Sleep apnea is caused, as mentioned, by a genetic mutation. A mutation is an error in the sequence of building blocks of our genetic code - DNA. Such errors typically occur during its duplication, when the repair mechanisms fail to prevent damage to the same sequence, resulting in an error in its reading and mistranslation of genetic information in the cell nucleus.
Mutations are classified into several types, such as - substitution of a nucleotide (the structural unit of DNA) in the sequence for another nucleotide, addition or deletion of nucleotides, and so on. Some mutations don’t cause a change in genetic expression (the phenotype), while others cause diseases, sometimes extremely dangerous and untreatable.
A specific type of mutation is expressed in the elongation of chains of the amino acid "alanine" - one of about twenty types of amino acids that constitute the building blocks of proteins in the body. This type of mutation causes nine different neurological diseases, including CCHS syndrome.
In this syndrome, the same mutation is expressed in the gene encoding the PHOX2B protein, which contributes to the normal formation and function of the autonomic nervous system, which controls unconscious processes such as breathing, digestion, heart rate management, thermoregulation, and more.
The new study into CCHS
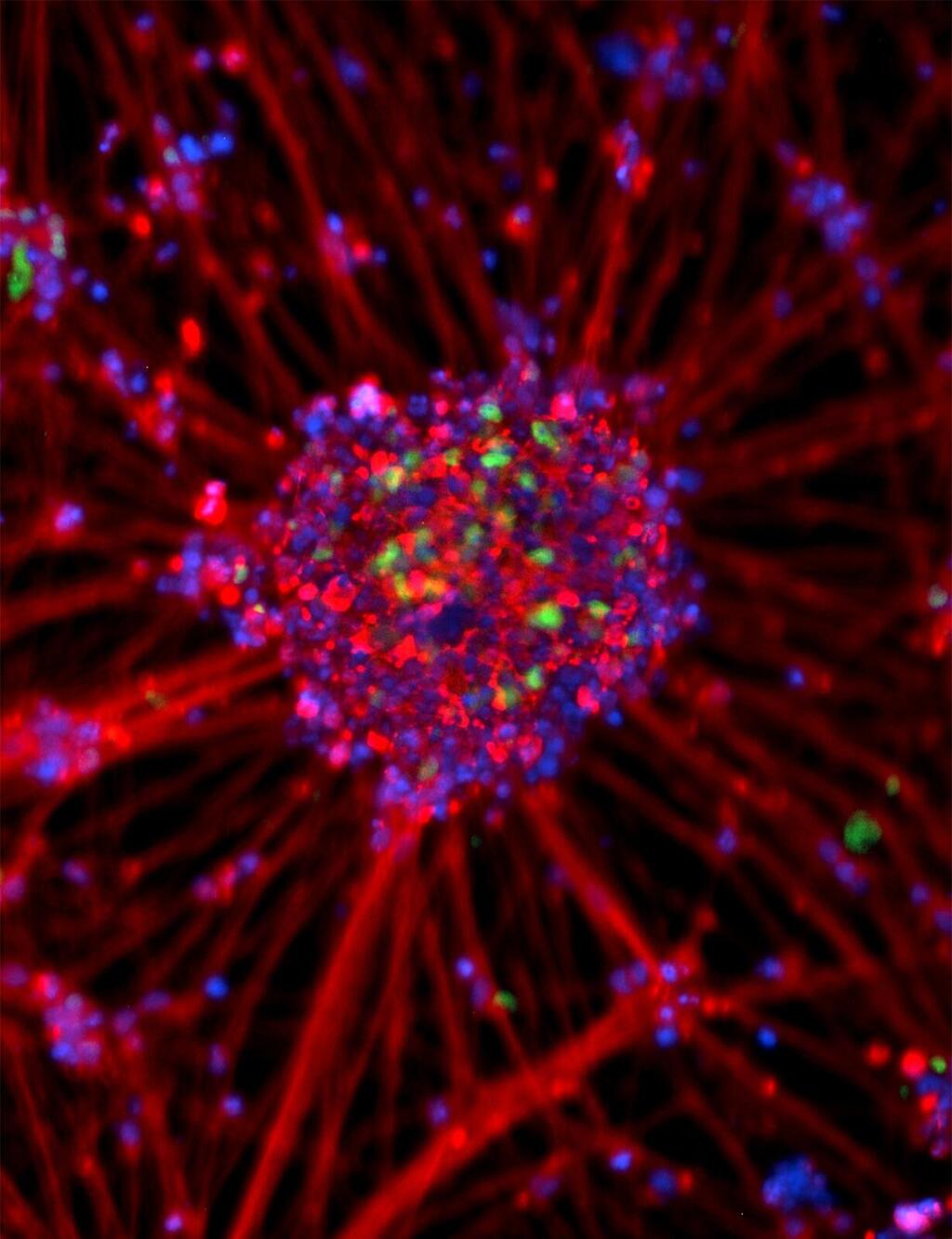
In order to investigate the behavior of the autonomic nervous system cells of CCHS patients, an innovative technique in the field of stem cells, called "reprogramming," was used. This technique was developed by the Japanese scientist Prof. Shinya Yamanaka, the Nobel Prize laureate in Physiology and Medicine for 2012, which allows relatively accessible cells (such as blood cells or skin cells) to be sent "back in time" to a state where they resemble embryonic stem cells.
Embryonic stem cells can split into all types of cells in the body, enabling the study of cells that are typically challenging to investigate in laboratory conditions, including nerve cells. In this way, researchers created stem cells from skin samples of CCHS patients.
These stem cells were then differentiated into autonomic nervous system cells whose function is impaired due to the syndrome, allowing the investigation of the nervous system’s function with the disease.
Through this method, impairment in the ubiquitin-proteasome system function associated with ubiquitin in the nerve cells of patients was discovered. The ubiquitin-proteasome system contributes to protein degradation in cells by tagging proteins destined for degradation.
This system was discovered by researchers Prof. Aaron Ciechanover, Prof. Avram Hershko, and Prof. Irwin Rose — who received the Nobel Prize in Chemistry in 2004 for their discovery.
Doctoral student Daniel Falik under the guidance of Prof. Gad Vatine and Fatma Amer-Zarzur under the guidance of Dr. Avraham Ashkenazi discovered that the mutation characterizing CCHS, causing elongation of the alanine chain in the PHOX2B protein, affects the access of that protein to the cell nucleus.
They found that the ubiquitin system is linked to the mutation in the PHOX2B protein that remains outside of the cell nucleus, thereby inhibiting the normal function of nerve cells. This inhibition leads to the death of nerve cells in the autonomic system and may cause the onset of CCHS.
Genetic therapy treatment
In addition to discovering the mechanism leading to the CCHS syndrome, the study also proposes a therapeutic approach with a potential cure - the use of genetic therapy. This therapy induces enhanced production of a specific enzyme (UBA6) in the ubiquitin system, thereby correcting the imbalance caused by the ubiquitin system's malfunction and thus preventing damage to autonomic nerve cells in CCHS patients.
- Prof. Gad Vatine from Ben-Gurion University is an expert in studying rare diseases using stem cells, Dr. Avraham Ashkenazi from Tel Aviv University specializes in diseases resulting from genetic mutations.