Getting your Trinity Audio player ready...
One of the main goals of Israel's Operation Rising Lion (“Am K’Lavi”) is to damage Iran’s nuclear program and thwart Iran’s ongoing efforts to produce enough fissile material for a nuclear bomb — a process known as uranium enrichment.
According to a report by the International Atomic Energy Agency (IAEA), as of May 2025, Iran possessed approximately 400 kilograms of uranium enriched to 60 percent, a level that likely brought it very close to having enough material for several nuclear bombs. What exactly is uranium enrichment? Why is it so difficult to carry out? And how is it connected to our ability to harness the vast energy stored within the atomic nucleus?
9 View gallery


The last element on the periodic table, found naturally in large quantities — and more importantly, a fissile one. A disc of enriched metallic uranium
(Photo: US Department of Energy)
Uranium fission
Uranium is a heavy, radioactive metal located near the bottom of the periodic table. Its atomic number is 92, meaning each atom has 92 protons in its nucleus. It is the heaviest naturally occurring element found in substantial quantities. Uranium is not particularly rare on Earth, and its supply is expected to remain accessible for many years, especially if extraction from seawater becomes viable.
What makes uranium unique is its ability, under certain conditions, to undergo nuclear fission—a process in which the atomic nucleus splits into the nuclei of lighter atoms, releasing energy. This is distinct from natural radioactive decay, which occurs in all radioactive materials and involves the emission of particles or electromagnetic radiation from the nucleus of an atom.
The atomic nucleus consists of two types of particles: protons, which carry a positive electric charge, and neutrons, which have no charge. The number of protons determines the element and defines most of its properties. The number of neutrons, however, is not fixed. An element can exist in different forms with varying numbers of neutrons in the nucleus—these are known as isotopes.
For example, all carbon atoms have six protons, and most also have six neutrons, making them carbon-12. Some carbon isotopes, however, have seven neutrons (carbon-13) or eight (carbon-14). Isotopes of the same element are nearly identical in their chemical properties but differ in certain physical characteristics. For instance, carbon-12 is stable, while carbon-14 is radioactive and decays over time. This allows scientists to date organic materials by measuring the ratio of carbon-12 to carbon-14 and calculating the material's age based on the decay rate of carbon-14.
Uranium-235 nuclei contain 92 protons and 143 neutrons. If a neutron strikes such a nucleus at the right speed, there is a high probability that it will split into two unequal parts, releasing a large amount of energy as radiation. The process also emits two or three additional neutrons from the fissioned nucleus. These neutrons may strike nearby uranium-235 nuclei, destabilizing them and causing them to split as well. In this way, the process continues as a self-sustaining chain reaction, releasing increasing amounts of energy. A material capable of sustaining such a chain reaction of nuclear fissions is known as fissile material.
9 View gallery
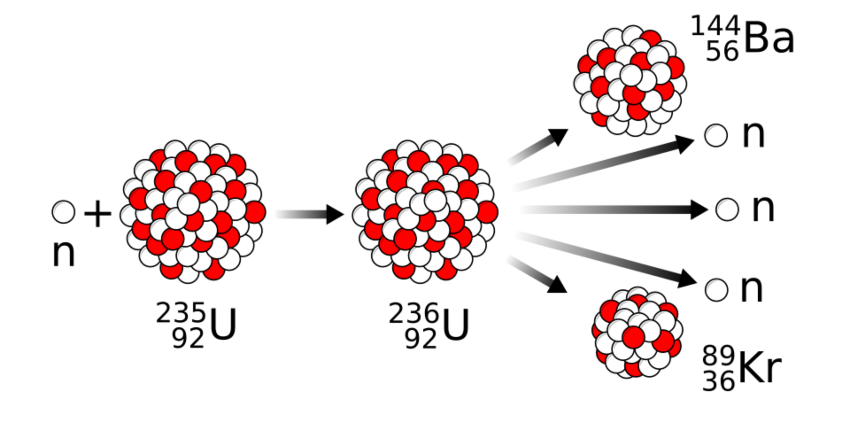

A process that releases immense energy: a neutron enters the nucleus of a uranium-235 atom, briefly forming uranium-236, which then splits into krypton and barium atoms, releasing three neutrons that can continue the chain reaction
(Illustration: MikeRun, Wikipedia)
However, uranium-235 makes up less than one percent of naturally occurring uranium atoms. Over 99 percent of natural uranium consists of uranium-238, which contains 92 protons and 146 neutrons. This isotope is only weakly radioactive. Although uranium-238 can undergo nuclear fission, it requires a collision with a highly energetic, fast-moving neutron. Furthermore, the neutrons released during its fission are not energetic enough to trigger additional fissions in other uranium-238 nuclei. As a result, this material cannot sustain the chain reaction necessary for a nuclear explosion. In other words, while uranium-238 is fissionable, it is not fissile, and therefore not suitable for initiating a nuclear blast.
With suitable fissile material, a chain reaction can escalate rapidly, resulting in a massive number of fissions in less than a millionth of a second, as in a nuclear explosion. Alternatively, the reaction can be initiated in a controlled manner and maintained at a relatively steady rate, as in nuclear reactors, where the energy released from fission is used to generate heat and produce electricity.
The speed and intensity of a chain reaction depend, among other factors, on the amount and concentration of fissile material. The higher the proportion of fissile atoms, the greater the likelihood that a neutron released during the fission of one nucleus will strike a nearby nucleus and trigger its fission as well.
Uranium enrichment: focusing on what matters
On the path to harnessing the vast energy stored in the uranium nucleus, a significant obstacle must be overcome. As noted, the overwhelming majority of natural uranium is uranium-238, an isotope that is not fissile and cannot sustain a nuclear chain reaction under any conditions. Only about seven-thousandths of natural uranium is uranium-235, the only naturally occurring fissile isotope.
This natural concentration is far too low to sustain a meaningful chain reaction, except in a few specialized reactor designs. To make the chain reaction more efficient, uranium must be enriched—that is, the concentration of the uranium-235 isotope in the uranium ore must be increased. This leads to two key questions: To what level should uranium be enriched, and how is the process carried out?
9 View gallery

Different applications require uranium with varying isotope concentrations. A small experimental nuclear reactor used in the Manhattan Project to develop the atomic bomb during World War II
(Photo: Los Alamos National Laboratory)
The answer to the first question depends on how the uranium will be used and the type of chain reaction desired. In nuclear reactors designed for electricity generation—where a steady, controlled release of energy is required, and a nuclear explosion is not— an enrichment level of just 3–5 percent is usually sufficient. Some reactor designs can operate using unenriched natural uranium.
Some reactors, such as fast breeder reactors, require a higher level of enrichment, though typically not exceeding 20 percent. Exceptions include compact reactors, like those used in submarines and large ships such as aircraft carriers, which often rely on uranium enriched to much higher levels—sometimes above 90 percent—since higher enrichment allows for a smaller reactor size.
Nuclear bombs require a relatively high level of enrichment. Uranium enriched to 20 percent or more is already considered weapons-grade, although building a bomb with uranium at that level is impractical and would require at least several hundred kilograms of material.
As a result, uranium intended for military use is typically enriched to over 90 percent uranium-235. For instance, the standard enrichment level for bomb-grade uranium in the United States is 93.5 percent. The atomic bomb dropped on Hiroshima in 1945 used uranium enriched to an average of about 80 percent, due to time constraints that prevented further enrichment. Had the same amount of uranium been enriched to 90 percent, the explosive yield would likely have been twice as high.
How is uranium enriched?
The second question — how to enrich uranium — is the more complex one. The chemical properties of uranium isotopes are nearly identical, making them unsuitable for separation by chemical means, unlike the isotopes of lighter elements. To separate uranium isotopes and remove as much uranium-238 as possible, we must rely on the tiny differences in their physical properties, particularly their mass. Even this is challenging, since the mass difference between uranium-235 and uranium-238 is less than 1.5 percent.
All uranium enrichment methods are based on this slight mass difference. Nearly all of them require uranium to be in gaseous form. To achieve this, uranium is combined with fluorine to form uranium hexafluoride (UF₆) — a corrosive and highly toxic compound, but one that can be easily converted to gas and processed. A key advantage is that fluorine has only one naturally occurring isotope, so any mass difference between UF₆ molecules is due solely to the uranium isotope they contain.
9 View gallery
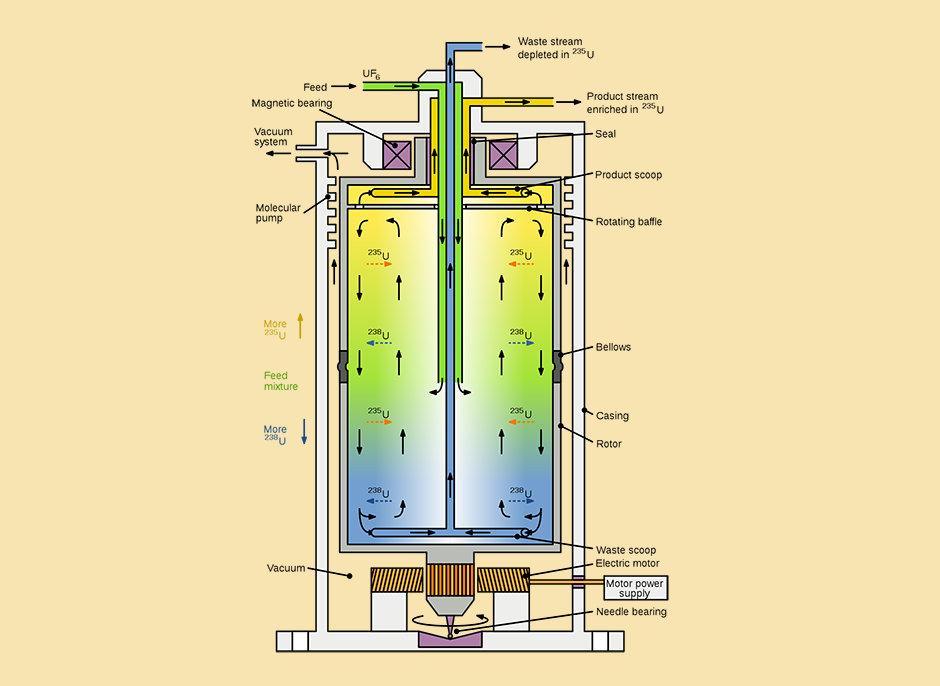

The most common and efficient method of uranium enrichment today uses centrifuges. The lighter isotope, uranium-235, tends to concentrate near the center of the spinning cylinder. Diagram of a gas centrifuge
(Photo: Wikipedia, Inductiveload)
The most common and efficient enrichment method in use today is gas centrifugation, which is also the method employed by Iran. In this process, UF₆ gas is fed into a long, cylindrical rotor, several meters in length, which spins at extremely high speeds — nearly 100,000 revolutions per minute. This causes partial separation: the heavier uranium-238 tends to move toward the outer edge of the cylinder, while the lighter uranium-235 concentrates closer to the center.
Another method, once widely used but now nearly obsolete, is gaseous diffusion. It relies on the natural process of diffusion, in which substances move from areas of higher concentration to areas of lower concentration. In gaseous diffusion, uranium hexafluoride (UF₆) gas is passed through a barrier with tiny pores. Because lighter molecules move slightly faster than heavier ones, the gas that passes through the barrier becomes slightly more enriched in uranium-235 compared to the gas that does not.
Both gaseous diffusion and centrifugation share a fundamental limitation: their separation efficiency is very low. The output is not pure uranium-235 or uranium-238 gas, but two streams — one slightly more enriched in uranium-235 than the original material, and one slightly less. This is due to the extremely small differences between the UF₆ molecules in both mass and speed.
For example, in gaseous diffusion, the velocity difference between UF₆ molecules containing uranium-235 versus uranium-238 is minimal. As a result, thousands of consecutive separation cycles are required to achieve significant enrichment. Centrifugation faces a similar challenge: a single centrifuge provides almost no meaningful enrichment. Substantial enrichment requires a cascade of thousands of centrifuges, with gas flowing from one unit to the next, becoming slightly more enriched at each stage.
This highlights another defining feature of all uranium enrichment methods: they are highly energy-intensive and costly. Centrifuges must spin at high speeds continuously, and the synchronization of thousands of units must be precisely maintained. In gaseous diffusion, the gas must be recompressed after each stage to maintain an effective diffusion rate.
9 View gallery
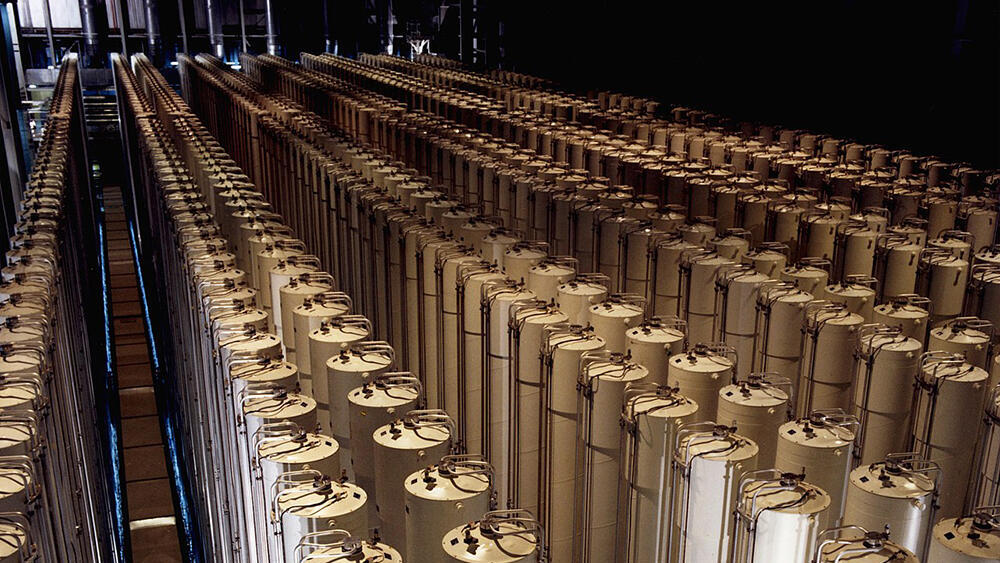

A centrifuge array at a uranium enrichment plant in Ohio, United States, during the 1980s. Modern centrifuges are shorter, but still measure several meters in height
(Photo: United States Department of Energy)
This method consumes roughly 50 times more electricity than centrifugation, making it far less economical. Uranium enrichment technology also involves numerous classified advancements, including the development of specialized materials used for manufacturing centrifuges that can withstand the extreme conditions necessary for efficient operation and for achieving enrichment levels suitable for military applications.
These are the two primary methods of uranium enrichment. Other techniques exist, but they are rarely used and certainly not on a large scale. Some — such as electromagnetic separation — are extremely costly and even more energy-intensive. Others, like laser-based separation, depend on technologies that are not yet fully developed.
A byproduct of the enrichment process is depleted uranium — almost pure uranium-238, containing only trace amounts of uranium-235. While it has little to no use in nuclear reactors or weapons production, it is less radioactive than natural uranium and relatively inexpensive, since it is produced as waste. Due to its high density, strength, and durability, depleted uranium has a range of civilian and military applications, including tank armor, radiation shielding, armor-piercing ammunition, and other uses that benefit from its chemical and mechanical properties.
Uranium enrichment around the world
Uranium enrichment is important for both military applications and for producing fuel used in the approximately 500 nuclear power plants operating worldwide. Most nuclear reactors require uranium enriched to less than five percent, which is why the vast majority of enriched uranium produced today falls within that range. Although this low-enriched uranium is not suitable for nuclear weapons, the technological leap from civilian to military-grade enrichment is considered relatively small, making it essential to regulate and monitor who can enrich uranium.
9 View gallery


As a byproduct, the enrichment process produces a significant amount of depleted uranium — a dense, strong, and relatively inexpensive metal Depleted uranium projectile
(Photo: Wikipedia, Choihei)
Today, nearly all nuclear-armed nations also possess uranium enrichment capabilities. Other countries — such as Germany, the Netherlands, and Japan — have the technical capacity to enrich uranium but do not use it for weapons development.
Russia is the global leader in uranium enrichment, accounting for about half of the world’s output. It is followed by Urenco, a British-Dutch-German consortium; a Chinese state-owned enterprise; and a French company with international partnerships. Together, these organizations produce more enriched uranium than the current global demand. This concentration of production in a few large, tightly regulated facilities is considered a key safeguard against the proliferation of nuclear weapons.
The five major nuclear powers — the United States, Russia, China, France, and the United Kingdom — currently produce little to no enriched uranium for military use and have largely ceased developing new nuclear weapons. However, their capabilities remain intact; during the Cold War, they amassed extensive stockpiles of warheads and have no immediate need to produce additional ones.
A significant portion of nuclear fuel used in recent decades has come from highly enriched uranium originally intended for weapons. After the weapons were dismantled, the uranium was diluted with natural uranium to reach the lower enrichment levels required for reactor fuel. This process is considered one of the major achievements in global nuclear disarmament, though it also undermined the economic viability of enrichment plants in the West and boosted Russia’s share in the global uranium supply.
When Pakistan began developing its nuclear program, it used centrifuges to enrich uranium. It is believed that a Pakistani scientist working at the European company Urenco in the 1970s obtained and stole designs for centrifuge construction and operation on behalf of his country. These stolen designs were reportedly later sold to other countries, including Iran.
9 View gallery


Russia is the world’s leading producer of enriched uranium. Gas centrifuges at a uranium enrichment facility in Russia
(Photo: Sputnik, Science Photo Library)
Uranium enrichment capacity is measured in Separative Work Units (SWU). Today, there are user-friendly tools available to calculate how many SWUs are needed to enrich uranium from one level to another, or alternatively, how much uranium ore is required to reach a specific enrichment level using a given number of SWUs.
The total global enrichment capacity currently stands at tens of millions of SWU. Enriching enough uranium to fuel a typical nuclear power plant at low levels requires over 100,000 SWU per year, and the majority of global uranium enrichment efforts are dedicated to this purpose.
While enriching uranium for nuclear fuel requires a significant energy investment, it is still far less than the energy a nuclear power plant will produce from that fuel. Enrichment accounts for about five percent of the cost of nuclear-generated electricity.
Although uranium enrichment for nuclear fuel requires a significant energy investment, it is still far less than the energy output of the nuclear plant using that fuel. Enrichment accounts for about five percent of the cost of nuclear-generated electricity. However, the high upfront costs make enriched uranium an expensive material. Uranium enriched to weapons-grade levels is especially expensive to produce, though its exact value is difficult to assess, as it is not traded on the open market.
Uranium enrichment in Iran
Iran’s nuclear program includes large-scale facilities for carrying out the various stages of uranium enrichment. According to publicly available sources, Iran converts uranium into uranium hexafluoride gas (UF₆) at a facility in Isfahan, which also serves as one of the country’s major nuclear research centers. This facility reportedly produces around 200 tons of UF₆ per year, which is then transported to Iran’s uranium enrichment plants — primarily the Natanz facility, considered the largest, and the Fordow facility, which is heavily fortified deep underground.
9 View gallery


As of May 2025, Iran was estimated to possess approximately 400 kilograms of uranium enriched to 60 percent. Uranium enrichment facility in Iran
(Photo: Digital Globe, Eurimage, Science Photo Library)
At these sites, according to published reports, Iran has managed to produce approximately 400 kilograms of uranium enriched to 60 percent fissile material. At this enrichment level, the remaining effort to reach weapons-grade enrichment (around 90%) by further centrifugation requires processing a smaller quantity of material, making the final steps relatively quick. Some estimates suggest that Iran could produce enough fissile material for one nuclear bomb within a few weeks, and the total quantity already enriched could be sufficient for up to ten bombs.
Get the Ynetnews app on your smartphone: Google Play: https://bit.ly/4eJ37pE | Apple App Store: https://bit.ly/3ZL7iNv
Iran has been developing its uranium enrichment capabilities for over 40 years. According to media reports, the Natanz facility was attacked during Operation Rising Lion and sustained some damage, despite being located aapproximately50 meters underground. The conversion plant in Isfahan was also reportedly attacked and damaged.
Enrichment of other elements
When it comes to uranium, the need for enrichment is clear. But what about isotope separation for other elements?
Isotope separation is performed for many elements, though typically on a small scale, usually just a few grams. For example, non-radioactive isotopes of carbon or oxygen are commonly used to label atoms in molecules for molecular biology research. Calcium has also been enriched in limited quantities for scientific studies, such as using one of its isotopes in the production of superheavy elements.
9 View gallery
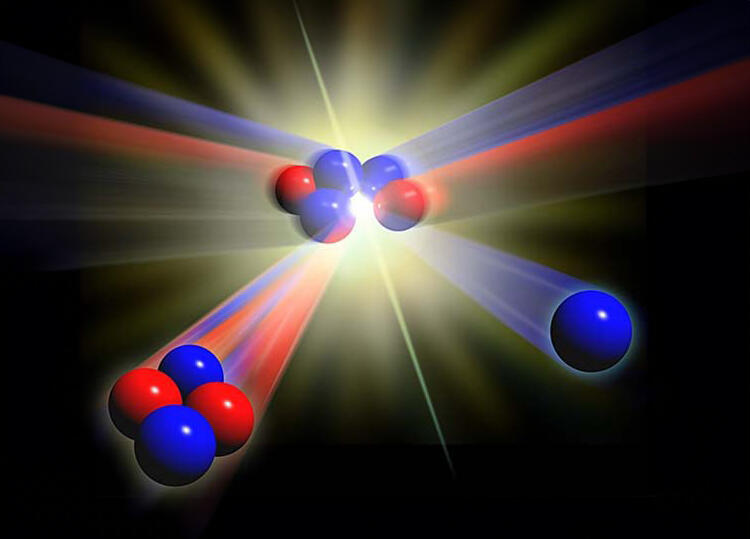

In the future, deuterium may be used in controlled nuclear fusion.Diagram of nuclear fusion: hydrogen-2 and hydrogen-3 collide to form a helium atom, releasing a neutron
(Photo: Seymour / Science Photo Library)
In recent years, silicon has drawn particular interest. Its enrichment yields silicon composed almost entirely of a single isotope, instead of the three naturally occurring ones. The crystal structure of isotopically pure silicon is more uniform, enabling superior thermal conductivity compared to natural silicon. This property is highly valuable in the semiconductor industry and may also be promising for future quantum computing technologies.
Large-scale isotope separation — involving thousands of kilograms or more — has been performed for only two other elements besides uranium: hydrogen and lithium. Lithium, the third element on the periodic table, is a lightweight metal widely used in rechargeable batteries as well as in thermonuclear (hydrogen) bombs. It has two naturally occurring isotopes, and the lighter isotope is more suitable for nuclear weapons applications, though it is relatively rare in nature. For this reason, nuclear powers enriched large quantities of lithium during the Cold War, but production ceased almost entirely after the Cold War ended. The large remaining stockpiles are now rarely used.
In the case of hydrogen, enrichment typically aims to isolate the heavy and rare isotope known as deuterium (hydrogen-2), which contains one proton and one neutron in its atomic nucleus, compared to the single proton in ordinary hydrogen (hydrogen-1). Deuterium is primarily used in the production of heavy water for nuclear reactors—water in which the hydrogen atoms are replaced by deuterium. Heavy water moderates (slows) the neutrons released during nuclear fission, increasing the likelihood they will be absorbed by other nuclei, thereby sustaining a chain reaction.
Deuterium is also a crucial component in hydrogen bombs, which rely on both nuclear fission and fusion reactions. As a result, countries with nuclear arsenals maintain large reserves of enriched uranium. Deuterium also has civilian applications, including in specific chemical analyses and biological research. In the longer term, it may play a role in generating clean and abundant energy, as it is a key ingredient in the fuel used for nuclear fusion.
The enrichment of hydrogen and lithium does not require centrifuges but instead relies on other techniques based on chemical differences between their isotopes.
This is feasible because in lighter elements, the relative mass difference between isotopes is much greater than in heavy elements like uranium, making their chemical properties more distinguishable.
Isotope separation, therefore, is a technology that has long played a role in military nuclear programs but also holds significant potential for civilian applications. In the future — perhaps within a few decades — when clean, affordable electricity from controlled nuclear fusion powers our homes, we may truly have come full circle: a technology once driven by the demands of war repurposed into a safe and inexhaustible source of energy for peaceful use.

