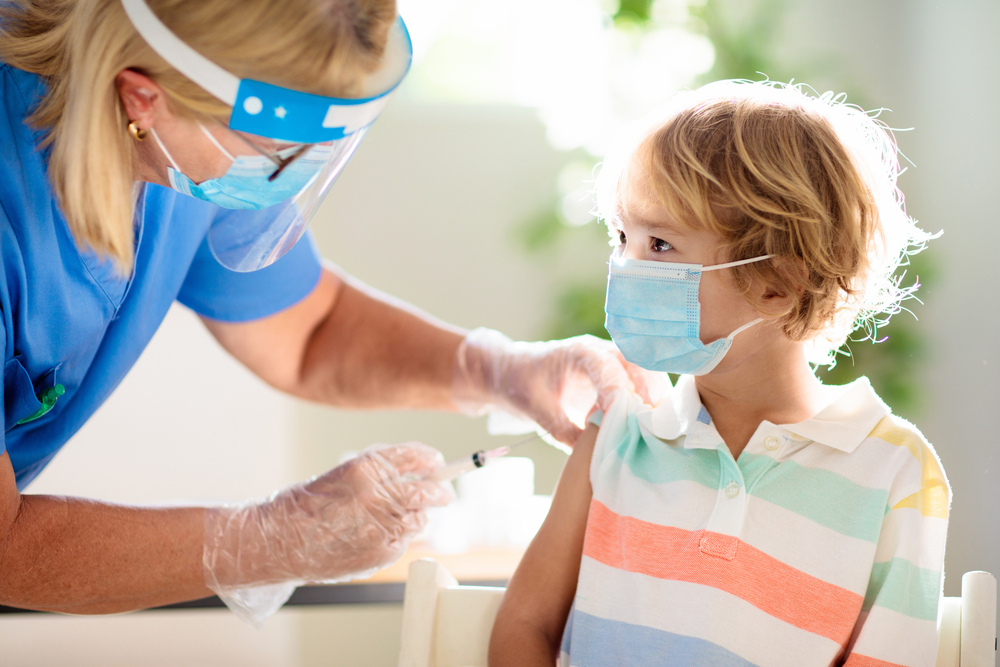
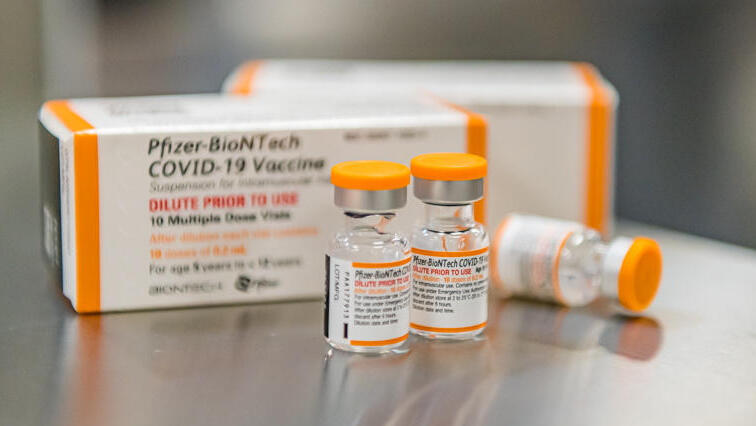
Following U.S. approval of coronavirus vaccines for children aged 5-11, the Health Ministry is said to be working to fast track a shipment of pediatric jabs to Israel in the coming days.
The vaccines' arrival will very much affect the schedule of the vaccine drive, which health officials said is set to kick off in the latter part of this month. A panel of health experts advising the government on the pandemic is set to vote Thursday on official approval of Pfizer vaccines on young children in Israel.
Officials are set to conduct the inoculation campaign through Israel's health funds rather than in schools, as was previously proposed by some officials.
This direction is affected by two key variables: the vaccination rate in schools of children aged 12-16 and the belief that parents would prefer to be near their kids during the vaccination procedure, which will be possible at clinics.
On Tuesday, the director of the U.S. Centers for Disease Control and Prevention (CDC) backed broad use of Pfizer's and BioNTech's COVID-19 vaccine in children ages 5 to 11, clearing the way for shots to go into young arms as soon as Wednesday.
The announcement comes hours after the advisers to the U.S. CDC unanimously supported the move, saying the benefits of the vaccine outweigh the risks. Much of their discussion stemmed from rare cases of heart inflammation that have been linked to the vaccine, particularly in young men.
The U.S. Food and Drug Administration granted emergency use authorization of the vaccine in 5- to 11-year-olds on Friday.
The FDA authorized a 10-microgram dose of Pfizer's vaccine in young children. The original shot given to those age 12 and older is 30 micrograms.
U.S. President Joe Biden called the authorization a "turning point" in the fight against the pandemic.