Getting your Trinity Audio player ready...
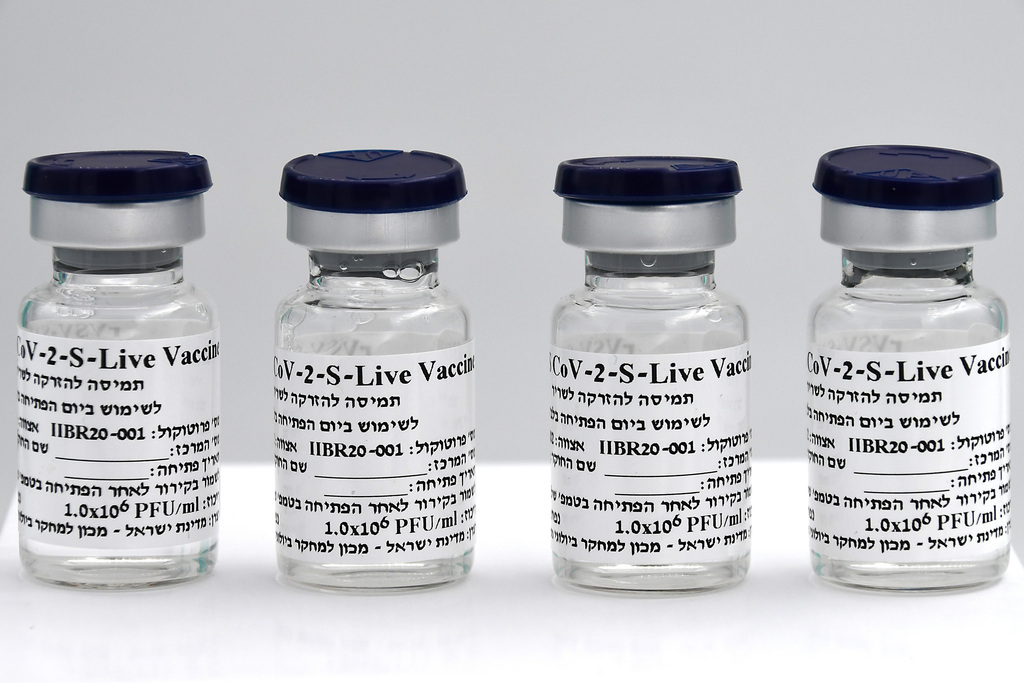
A shipment of some 1,000 vials of the Israeli-developed BriLife coronavirus vaccine was sent over the weekend from Israel to Georgia, where the second phase of its clinical trials is taking place, the Ministry of Defense announced Sunday.
Last month, The Israel Institute for Biological Research (IIBR), which is developing the vaccine, signed a memorandum of understanding with the U.S.-based pharmaceutical company NRx that includes the worldwide development, manufacturing and marketing rights of the vaccine, which has been undergoing early clinical trials in Israel in recent months.
NRx said it has arranged for rapid Phase 2b/3 testing in Israel, Georgia and Ukraine. The Defense Ministry, which oversees the biological research institute, said the trials will include tens of thousands of volunteers.
BriLife is a self-propagating, live-virus vaccine, which sets it apart from mRNA vaccines produced by companies like Pfizer, which are currently being used in Israel's successful vaccination campaign. It will initially be delivered via injection, NRx said.
None of the vaccines authorized for use in the United States contain a live virus, according to the Centers for Disease Control.
2 View gallery


Israeli volunteer Segev Harel receiving a dose of the Israeli-developed BriLife vaccine
(Photo: Defense Ministry)
There had been some discussion in Israel about whether the locally developed vaccine was necessary after the country opted to import Pfizer's en masse. Officials said it was worth keeping the project going in order to ensure a locally made alternative was eventually available.
The IIBR aims to produce 15 million doses once the development is completed.