Getting your Trinity Audio player ready...
Glass is one of the most useful materials available to humans. It is used in construction, automotive industries, utensils, mirrors, eyeglasses—almost everywhere. While it’s tempting to attribute its existence to human ingenuity, in this case, nature beat us to it. It turns out that meteor impacts on sandy surfaces and volcanic eruptions can create the right conditions for natural glass to form. Volcanic glass, known as obsidian, was used in ancient times to make knives and weapons.
Glass is classified as an amorphous solid, which means that unlike most solids, its atomic structure is very disordered, resembling more that of a liquid than that of a solid. It forms when molten sand cools very rapidly, preventing the molecules from organizing.
2 View gallery
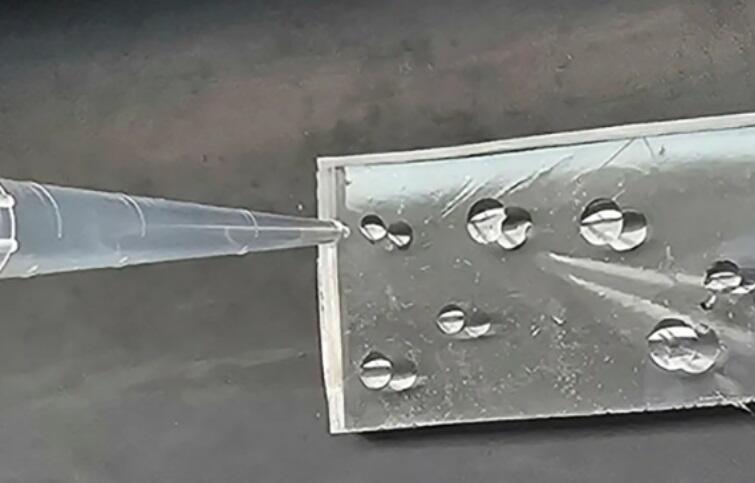

A glassy material with adhesive properties that can self-repair cracks. Tri-Tyrosine
(Photo: Tel Aviv University Spokesperson's Office)
Glass manufacturers produce glass in a similar process, by melting beach sand (primarily silica), shaping it as desired, and then cooling it quickly, often in lukewarm water. This rapid cooling creates a sharp transition from the flexible molten state to the hard glass, known as the "glass transition"—a type of thermodynamic phase transition, akin to changes in states of matter.
Great Promise
Glass is an incredibly useful material—transparent, hard, and fairly resistant to environmental damage and wear. However, it is brittle and cannot be reverted back to sand. These limitations present a challenge to materials scientists, who are searching for ways to create new materials that are similar to glass but with enhanced properties.
Alongside these efforts, materials scientist Professor Ehud Gazit from Tel Aviv University, together with his students Gal Finkelstein-Zuta and Zohar Arnon, have made a particularly promising discovery. In their research, published in the journal Nature, they report the discovery of a glass-like substance formed through the spontaneous self-organization of a material known as tyrosine tripeptide (YYY). While this new material closely resembles glass, it also exhibits unique properties The significance of their discovery is underscored by the journal's choice to feature a “News and Views” article in the same issue, which discusses the potential implications of their findings, as well as by a video detailing the new material's properties.
Tyrosine is one of the twenty amino acids that make up all proteins in living organisms. Tyrosine tripeptide is a short protein chain composed of three tyrosine units. "The biochemical abbreviation for tyrosine tripeptide is YYY, which may hint at its intriguing properties," Finkelstein-Zuta explained in an interview with the Davidson Institute's website. "We discovered, quite by chance and during other experimental attempts, that an aqueous solution of this peptide allows for the creation of a glassy material—remarkably transparent, even more so than silicate glass. Additionally, this new glass can change its mechanical properties, repair cracks that form in it, and it also possesses adhesive properties."
2 View gallery


Displaying a plate made out of the peptide glass. Gal Finkelstein-Zuta and Professor Ehud Gazit
(Photo: Tel Aviv University Spokesperson's Office)
Perhaps the greatest advantage of this glass is its simple production process, which Finkelstein-Zuta compares to making raspberry syrup in a home kitchen. All that’s required is to mix tyrosine tripeptide powder with water, and that’s it. This contrasts sharply with the production of silicate glass, which demands considerable energy investment and specialized skills accumulated over many years of training. The new glass forms spontaneously, without any energy input.
With A Little Help from Friends
The researchers employed various methods to characterize the material they discovered. To analyze its optical properties, they collaborated with Prof.Tal Ellenbogen from the School of Electrical Engineering at Tel Aviv University. They found that it is transparent not only in visible wavelengths but also in the infrared range, which could potentially allow its use in data communication. To characterize its molecular structure, they consulted chemist Prof. Amir Goldbourt from Tel Aviv University. Additional characterizations of the material’s adhesive properties and mechanical characteristics were conducted with assistance from Dr. Maxim Sokol from Tel Aviv University and Dr. Ben Palmer from Ben-Gurion University. They discovered that Tri-Tyrosine, when used as an adhesive, can support a load of up to five kilograms.
The most surprising feature of the new peptide glass is its ability to "heal" itself, providing a significant advantage over traditional silicate glass. "We found that although the peptide glass cracks under dry conditions," Finkelstein-Zuta explained, "it can easily repair these cracks. All that needs to be done is to transfer the glass to a more humid environment. As soon as you do that, water molecules re-enter the material, repair the cracks, and allow the glass to reform. This happens because water molecules act as connector joints between the tyrosine molecules; when these joints break due to water loss, they can be restored, allowing all connections to be reformed."
The most surprising feature of the new peptide glass is its ability to "heal" itself, providing a significant advantage over traditional silicate glass. "We discovered that although the peptide glass cracks under dry conditions," Finkelstein-Zuta explained, "it can easily repair these cracks by being moved to a more humid environment. Water molecules re-enter the material, repair the cracks, and allow the glass to reform. The reason is that the water molecules act like joints that connect the tyrosine molecules; when the joints break due to the loss of water, they can be restored, allowing all the connections to be reformed."
Gazit is optimistic about the technological and industrial applications of the new material, even suggesting that it could pave the way for an entirely new research field
"It will take some time before we can produce eyeglasses based on tyrosine," Finkelstein-Zuta concludes. "We’ve introduced a new 'baby' to the world, named it, but we still don’t know what it will become when it grows up. For now, we’ve managed to produce lenses based on the peptide glass in the lab, and I look forward to follow-up research that will take our discovery further." It's transparent that this innovation has great potential.

