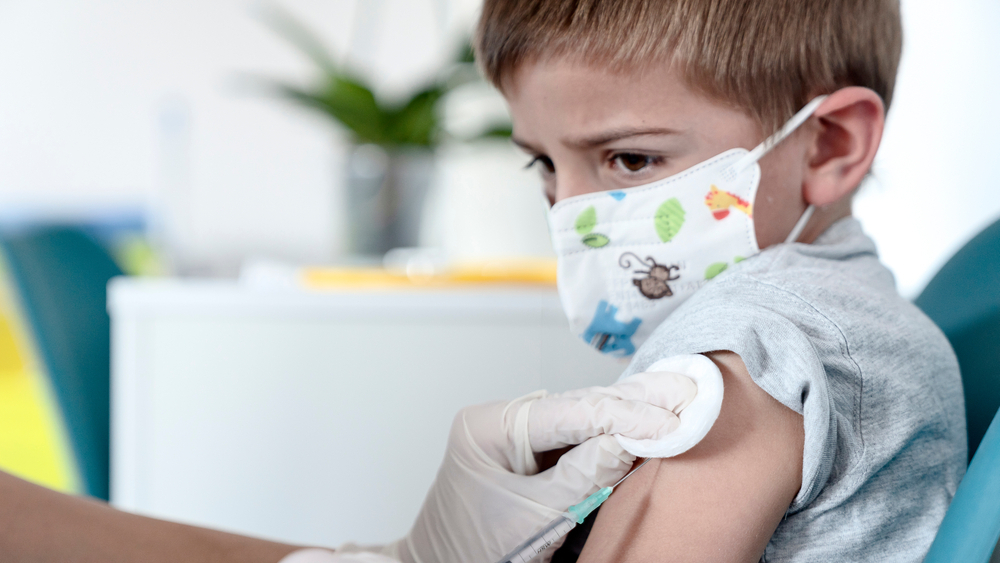
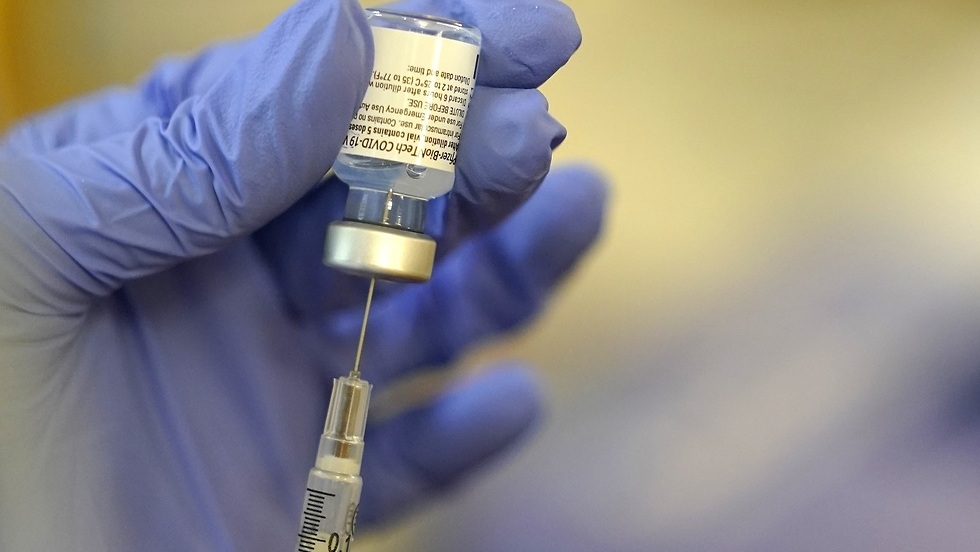
A panel of health experts advising the government on the pandemic on Thursday began deliberations to weigh the administration of pediatric coronavirus vaccines to children aged 5 to 11.
Deliberations will be held publicly and carried live on Ynet as well as other media outlets as of 3 pm local time. The officials said they wanted to increase confidence in their decision-making process and allow Israelis to ask questions and voice their concerns.
On Wednesday, the U.S. Center for Disease Control announced it is recommending pediatric COVID-19 vaccines for children aged 5 to 11. The CDC followed a decision by the American Food and Drug Administration that the vaccines should be administered.
The deliberations will include senior physicians and researchers as well as representative of the public.
At the start of the meeting, officials will present a current picture of morbidity from COVID-19 among young children, including hospitalizations, serious illness and the death of a 16-year old last month following an organ failure while infected by the virus.
Participants will also be presented with Pfizer research and the FDA and CDC decisions to recommend pediatric vaccines, based on their data. They will also be given data collected around the world and from the vaccine producing pharmaceutical companies.
The health officials will devote a substantial portion of the time to possible - though rare - side effects of the vaccines, including myocarditis. Pfizer reported that its data showed no cases of the heart complication was reported in the 5-11 age group.
Health Ministry Director General Prof. Nachman Ash told Ynet on Thursday that he estimates the vaccines will be authorized for use in Israel. "The occurrences of adverse side affects of the vaccines are fewer than the complications caused by the virus," Ash said.
He added that the Health Ministry was coordinating with the Education Ministry on the administration of the pediatric vaccines in schools.
First published: 14:40, 11.04.21