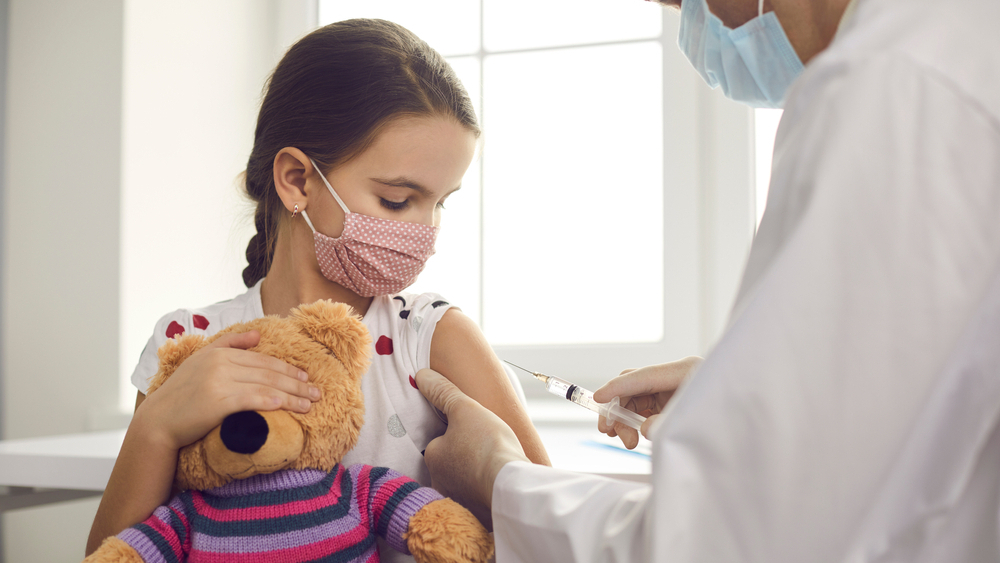
A high-level medical panel of U.S. government advisors will meet Tuesday to decide whether to authorize the Pfizer COVID-19 vaccine for children aged five to 11 years old.
If the independent experts convened by the U.S. Food and Drug Administration (FDA) will vote in favor, an emergency authorization of the vaccine could follow within weeks.
This would make 28 million younger children eligible for the shots this coming November.
During the meeting, the expert panel will have to rely on scientific evidence in order to decide whether the benefits of the two-dose vaccine, given three weeks apart, outweigh the known risks?
Ahead of the meeting, the FDA uploaded an analysis by Pfizer that showed the vaccine was 90.7% effective at preventing symptomatic COVID infections with no serious safety issues.
The FDA also posted its own briefing document containing a risk-benefit analysis, which indicated the agency's scientists believe the benefits exceed the most worrying potential side-effect for the younger age groups: Myocarditis, also known as inflammation of the heart muscle.
"My initial thought is that the benefits of vaccinating children five through 11 years outweigh the risks of myocarditis and other safety concerns that people may have," Henry Bernstein, a pediatrician at Cohen Children's Medical Center in New York, said.
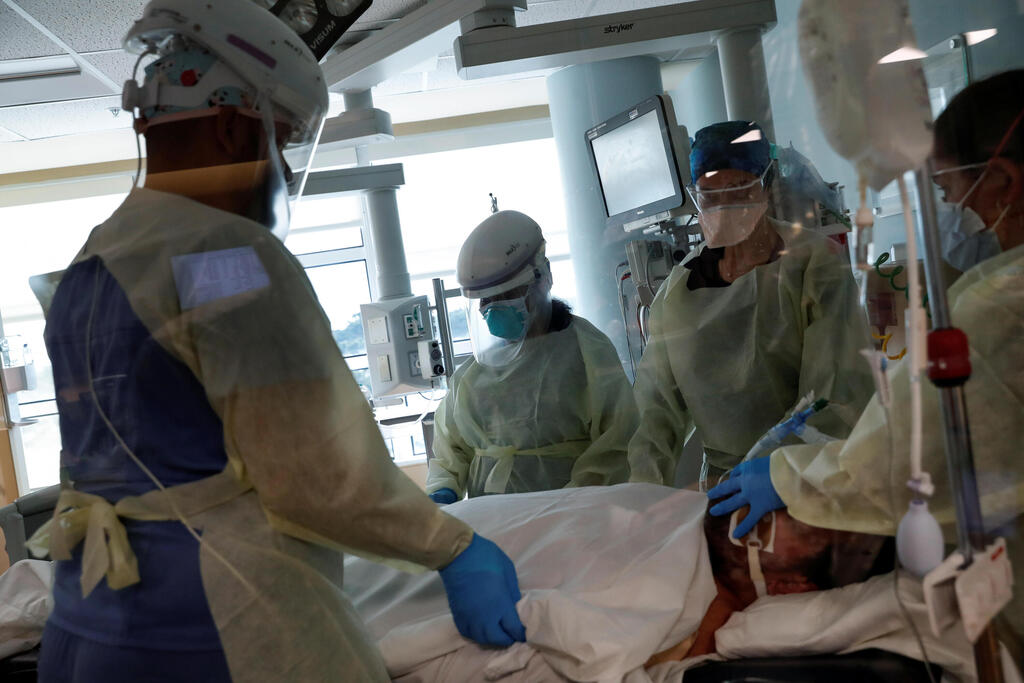
Overall, 160 children aged 5-11 have died from COVID in the United States - a tiny fraction of the total U.S. COVID death toll, which currently stands at over 730,000.
There have also been more than 5,000 cases of multisystem inflammatory syndrome in children (MIS-C), a rare but highly serious post-viral complication, which has claimed 46 lives.
According to Pfizer's evaluated safety data though, gathered from a total of 3,000 vaccinated participants, the most common side-effects are mild or moderate, including injection site pain, fatigue, headache, muscle pain and chills.
Pfizer added that while there were no cases of myocarditis or pericarditis (inflammation around the heart) during its clinical trial, there were not enough study volunteers to be able to detect highly rare side-effects.
Very rare instances of myocarditis were only detected in adolescents after the vaccine was authorized in June and given to millions of people in that age group, rather than the thousands that were tested in trials.
Scientists believe it will be even rarer among younger children, but don't expect to know just how rare until it is green-lighted.
The FDA acknowledged that, hypothetically speaking, if COVID-19 transmission was crushed within communities -- as was the case last in June 2021 -- the number of vaccine-induced myocarditis cases could exceed the number of COVID-19 hospitalizations prevented.
But even then, it added, the benefits might still exceed the risks, because non-hospitalized COVID-19 cases can have more serious consequences than side-effects, which are normally temporary.