Getting your Trinity Audio player ready...
The 2022 Academy Awards ceremony will be primarily remembered not for its winners and losers, but for the dramatic incident that unfolded during the entertainment segment of the evening. Reacting to a distasteful joke made by the host, comedian Chris Rock, about the shaved head of actress Jada Pinkett-Smith, her husband, actor Will Smith, came on stage and slapped Rock in the face. Smith later explained that he lost his temper and reacted with inappropriate violence to the insult to his wife, who suffers from the skin condition alopecia areata, a disease characterized by localized hair loss.
More stories:
Some might underestimate the impact of skin conditions, perceiving them as merely aesthetic issues. However, alopecia areata, commonly referred to as "spot baldness," frequently imposes a profound psychological and social burden on those affected by it, significantly undermining their quality of life and self-esteem.
Patients experience sudden hair loss, affecting the scalp, facial hair – including eyebrows or beard, and occasionally body hair. This condition is caused by an autoimmune disease – a state in which the body’s immune system erroneously attacks its own healthy cells. In individuals with alopecia areata, the hair follicles in the skin, from which hair grows, become the target of this attack. Consequently, hair sheds, leading to small bald patches the size of a coin, which sometimes spread across large areas of the scalp or beard.
The disease is relatively common, affecting about 2% of the population, irrespective of gender. Although it can occur at any age, it often tends to first appear at a younger age. On average, three out of every five patients will experience spot baldness before the age of 20, and one will develop it in their early adult years before the age of 40.
The damage to the hair follicle is reversible, since despite the hair loss, the follicle itself remains viable and may eventually regrow new hair. Indeed, hair regrowth typically occurs naturally within about a year, resulting in the disappearance of the bald patch. However, the disease itself has no cure, and may recur, possibly affecting even those areas that had previously recovered.
Despite being a particularly common autoimmune disease, the underlying mechanisms causing it are not fully understood, and the available treatments remain inadequate. Treatments involving corticosteroids, administered orally, via injection or through topical applications such as ointments, suppress the activity of the immune system and reduce the inflammatory response. Other ointments stimulate hair regrowth in the bald areas, and in severe cases certain ointments are used with the goal of resetting the immune system to prevent it from attacking the hair follicles. However, these treatments have limited effectiveness in moderate to severe cases and can carry distressing side effects.
A new treatment
Up until now, no treatment had received full approval from the US Food and Drug Administration (FDA). In 2022, Baricitinib, a drug developed to mitigate the body's inflammatory response, received approval from both the FDA and the European Health Agency. However, this approval was limited for use only in adults suffering from the severe form of the disease.
The final and decisive stage of a clinical trial for the new drug, Ritlecitinib, has recently been completed successfully. This trial, funded by the pharmaceutical company Pfizer, is detailed in an article published in The Lancet journal. The drug disrupts the activity of proteins essential for the function of immune system cells that attack hair follicles. The research was designed to assess how beneficial the drug is for patients and to identify its major side effects, with the objective of evaluating its effectiveness and safety for various doses.
2 View gallery
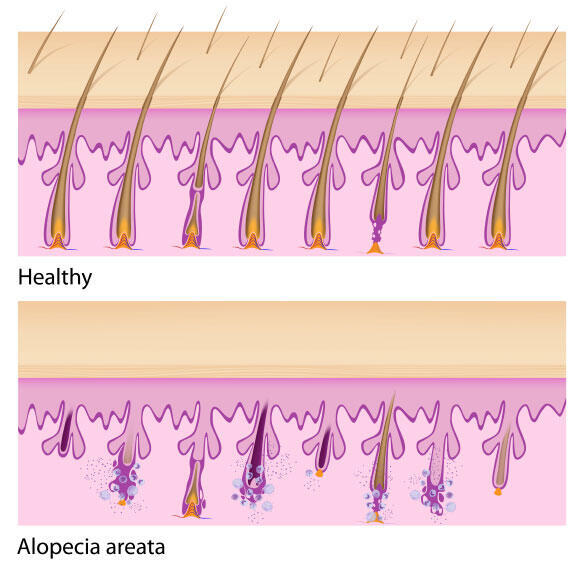

Healthy hair follicles (upper panel) and hair follicles damaged by alopecia areata (lower panel)
(Photo: Alila Medical Media, Shutterstock)
The trial took place at 118 hospitals and clinics in 18 countries, involving a total of 718 patients with a diagnosis of alopecia areata, ages 12 and up, who had suffered at least 50% scalp hair loss. Over 100 of them were adolescents ages 12-17 years.
Patients were randomly divided into several groups, each receiving different doses over a 48-week trial period. For the control, two groups were administered a placebo for the first 24 weeks, then switched to two different doses of the actual drug for the remaining 24 weeks.
The results revealed that both the duration of treatment and the size of the dose positively influenced outcomes. By the midpoint of the trial, 31% of patients receiving the highest dose experienced complete or near-complete regrowth of scalp hair, compared to just 2% in the control groups. By the end of the second half of the trial, the percentage of patients in the first group who experienced hair regrowth increased to 40%.
Encouraging findings
Regarding the drug's safety, most participants experienced only mild-to-moderate side effects, such as upper respiratory tract inflammation and headaches. Notably, there were no reports of patients experiencing severe side effects such as cardiac events, infections or fatalities. These findings are encouraging and demonstrate that the use of Ritlecitinib for patients with alopecia areata is an effective and safe option for both adults and adolescents.
The lead author of the study, Brett King, an associate professor of dermatology at Yale School of Medicine, asserted that: “This new work is a huge advancement for treating alopecia areata because the clinical trial involved adolescents in addition to adults. Because alopecia areata frequently affects children and adolescents, it is groundbreaking to advance a medicine that shows safety and effectiveness in the treatment of younger patients,” he said.
King further highlighted the significant distress caused by the condition. “Alopecia areata often causes enormous suffering, for adults and kids alike. Being a kid is hard enough as it is, so imagine what it’s like to be a kid with big bald spots, maybe missing an eyebrow, maybe without any hair at all. It can be punishing.”
Research on Ritlecitinib is ongoing, with investigators now focusing on the long-term effects of the treatment. The encouraging results from the current trial substantially enhance the likelihood that this medication could offer an effective solution in the future for patients suffering from alopecia areata, including the younger patients who often endure a particularly heavy social burden due to their condition.
Content distributed by the Davidson Institute for Science Education


