Getting your Trinity Audio player ready...
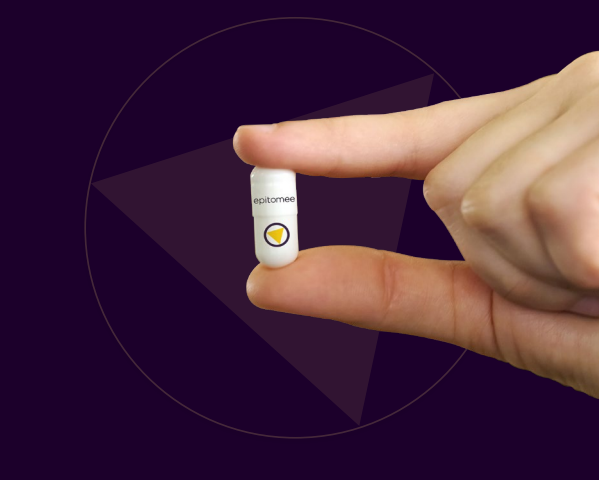
The Biomed company Epitomee's share value spiked this month (188%) after its new weight loss pill was approved by the FDA. The pill, which inherited its company's name will join the fierce competition between weight loss pills in the market.
In complete contrast to Novo Nordisk's Wegovy and Eli Lilly's Mounjaro, Epitomee's capsule does not contain any active substance. It is a sophisticated pill that, after being swallowed and arriving at its intended destination, expands and changes its shape into a rigid triangle, in response to the acidic environment. In doing so, it mimics the presence of solid food, which causes the stomach to feel full and activate the brain's sections of feeling satisfaction after eating. The pill lasts a few hours in the stomach and later moves to the intestines to break down within a few minutes.
Epitomee is far from the only company trying to challenge the weight loss drug market, which currently relies mainly on injections rather than orally ingested drugs. After all, it is no secret that the majority of people who need medical treatment would prefer to take pills or capsules over injections, mainly because it is more natural and comfortable. This fact is backed by studies and doctors who prefer to prescribe a pill over an injection.
Another advantage that may tip the scales in favor of taking a pill over an injection is related to the patient's ability to control the side effects. Certain patients, who develop intolerable or dangerous side effects as a result of a drug can benefit from an immediate cessation of these effects as soon as they suspend the daily oral treatment. However, patients who take weekly injections are unable to cease the effects of the drug so quickly.
This data is useful both economically but also medically, since ingesting a pill on a daily basis promotes medical adherence and persistence with the treatment. Those who take oral medications for weight loss are expected to benefit more healthily in the long term, compared to those who are treated with injections.
 Dr. Lior NeumanPhoto: Hila Shahar
Dr. Lior NeumanPhoto: Hila ShaharIn the Israeli market, there are currently only two drugs that are taken orally and are used to treat obesity. Both are quite old and usually result in weight loss, but this loss is not as dramatic as one would expect with the new injections. The first drug is Razin, a capsule that is taken once a day, contains the active substance and appetite suppressant phentermine, and contributes to a weight loss of about 7% in about six months with potential side effects of dry mouth and insomnia. A more effective drug with phentermine-topiramate is quite popular in the United States but is not available in Israel.
The second drug available in Israel is Orlistat, a drug containing the active ingredient xenical, which acts on the digestive system and interferes with the absorption of fats. The result is a relatively modest reduction in weight of about 3 kg, with a price of relatively common side effects.
The drug market for the treatment of overweight and obesity, which is heavily dominated by weight loss injections, is being joined by three pills, which may also arrive in Israel in the coming years.
Wegovy in a pill
Who hasn't heard of Rybelsus? It is an innovative oral medicine, also produced by Novo-Nordisk, which contains the active substance semaglutide which is found in Ozempic and Wegovy injections. This substance is the so-called "GLP-1 receptor agonist" a protein similar in structure to the human GLP1 hormone, that connects to the receptors of this hormone, which are scattered in different places in the body, thus causing different beneficial effects. When semaglutide binds to receptors in the brain, it suppresses hunger and increases satiety.
However, this is a pill intended solely for the treatment of type 2 diabetes, and not for the treatment of obesity. The weight loss caused by the pill is relatively modest (around 4 kg). The reason is that the maximum dose of the active substance present in this pill is small (14 mg) and corresponds, more or less, to a dose of between 0.5 mg to 1 mg of the substance given by injection.
To maximize the Wegovy injection's effect, which contains 2.4 mg of the active substance, Novo Nordisk realized a pill would have to be several times bigger. About a year ago, the Lancet medical journal published the results of the OASIS study that tested the weight loss potential of a new pill containing no less than 50 mg of semaglutide. The study showed a 15% weight loss in 68 weeks, similar to the Wegovy injection, but this pill has not yet received FDA approval.
The pill seems to be effective only if it is ingested on an empty stomach with a little water. Then, the patient must wait for at least half an hour before eating or taking other medication.
Orforglipron
This is Eli Lilly's pill which competes with Novo Nordisk's pill. The active substance in the pill is a partial agonist for the GLP1 receptor, but it is a more compact protein that is called a non-peptide. According to the results of the small phase II study, published last year in the prestigious New England Journal of Medicine, the pill results in a dose-dependent weight loss of up to 14.5% after 36 weeks. Eli Lilly is currently working on conducting the third phase of the study, so the pil is not expected to progress in the near future.
Amycretin
This is another promising development coming from Novo Nordisk. The active substance present in the amicertin pill actually works twice as hard, because it connects not only to the receptors of the GLP1 hormone, but also to the receptors of amylin. The amylin hormone is physiologically produced in the pancreas, and when it is absorbed into the central nervous system, it also contributes to diminishing hunger.
The small study (about 120 participants), whose main purpose was to test the safety of the drug in different doses and against a placebo, demonstrated a promising weight loss of 13% within 12 weeks - twice what is known about Wegovy in the same period of time. The side effects were, as expected, mainly related to the digestive system, and were usually mild to moderate in intensity, relatively tolerable and dose dependent. The results of the larger and longer study will only be revealed during 2026.
The reality test
All the discussed pills have not passed the real world test, which is ultimately the most decisive of all. Various pills for obesity have already received FDA approval recently, and then were panic-stricken from the market's fear of dangerous side effects. The wait to find a miracle pill could take years. For now, it is crucial to engage in the basics: physical activity and eating healthy.
Dr. Lior Neuman is an expert in family medicine and treats patients for obesity and diabetes.