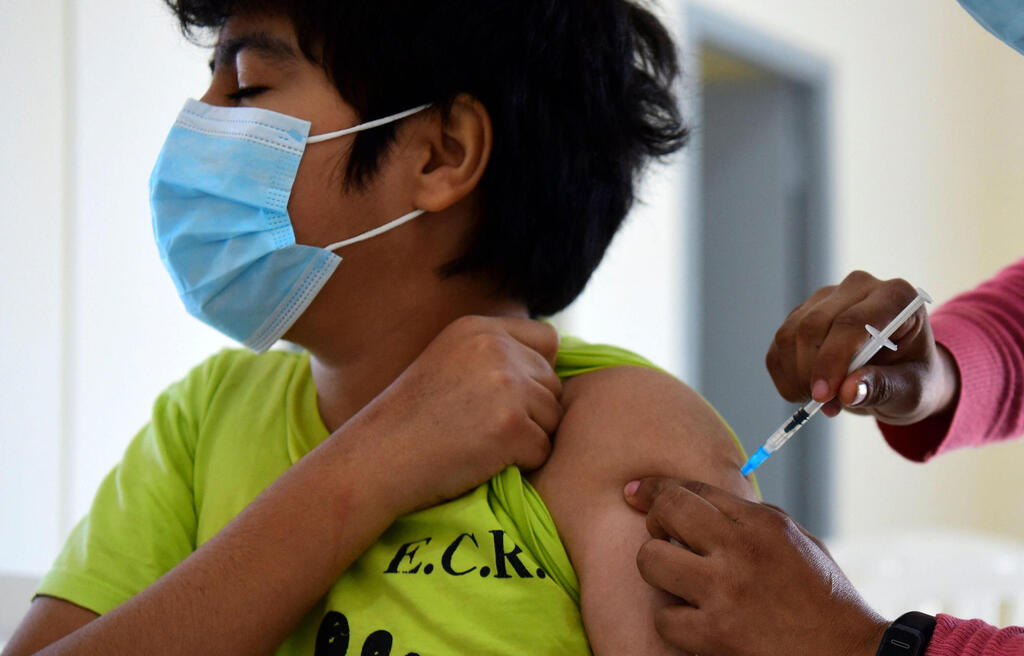
Pfizer Inc and BioNTech SE on Tuesday submitted initial trial data for their COVID-19 vaccine in children aged 5 to 11 and said they would make a formal request with U.S. regulators for emergency use in the coming weeks.
Coronavirus infections have soared in children, hitting their highest point in early September, according to data from the American Academy of Pediatrics.
The vaccine, which is already authorized in teens aged 12 to 15 and fully approved for ages 16 and up, induced a strong immune response in the target age group in a 2,268-participant clinical trial, the companies said on Sept. 20.
The Pfizer-BioNTech vaccine was authorized in kids aged 12-15 roughly a month after the companies filed for authorization. If the same timeline is followed for this application, younger children could start receiving their shots as soon as late October.
A rapid authorization could help mitigate a potential surge of cases this fall, with schools already open nationwide.
While kids are less susceptible to severe COVID-19, they can spread the virus to others, including vulnerable populations that are more at risk of severe illness.
The companies said they plan to submit the data to the European Medicines Agency and other regulatory authorities.
Data from the companies' trial showed the two-shot vaccine generated an immune response in children that matched what was previously observed in 16-to-25 year olds. The safety profile was also comparable to the older age group, Pfizer said.
The drugmakers are also testing the vaccine in children aged 2-to-5 and those aged 6 months-to-2 years, with data expected in the fourth quarter.
Moderna's COVID-19 vaccine is not yet authorized for use in adolescents in the United States, while it has gained authorization for that age group in Europe.