Getting your Trinity Audio player ready...
The European regulator on Monday approved a third dose of the Pfizer/BioNTech COVID-19 vaccine for those over 18, fearing that vaccine protection against the disease may wane as time goes by after completing the original two-injection regimen.
The European Medicines Agency (EMA), headquartered in Amsterdam, also approved supplementary jabs of Moderna and Pfizer vaccines for people with severely weakened immune systems.
2 View gallery
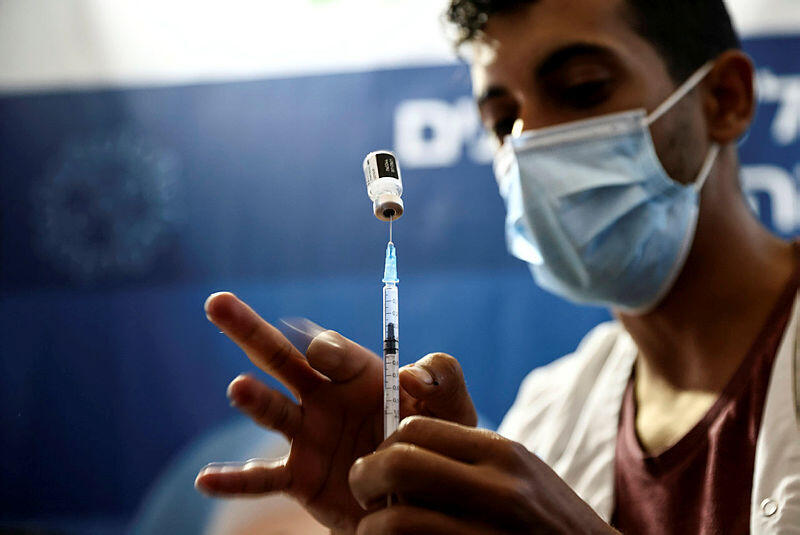

Health worker holds a vial of the Pfizer/BioNTech COVID-19 vaccine and syringe at a clinic in Jerusalem
(Photo: Reuters)
"Booster doses of Comirnaty (Covid-19 mRNA vaccine) may be considered for people aged 18 and over, at least six months after the second dose," the EMA said in a statement, referring to the trade name of the Pfizer vaccine.
"Decisions on third doses will be made by public health bodies at the national level," the EMA said.
The EMA's Committee for Medicinal Products for Human Use (CHMP) has "evaluated data for Comirnaty showing an increase in antibody levels when a third dose is given."
"The risk of inflammatory heart disease or other very rare side effects after a booster is not known and is carefully monitored," the EMA added.
2 View gallery
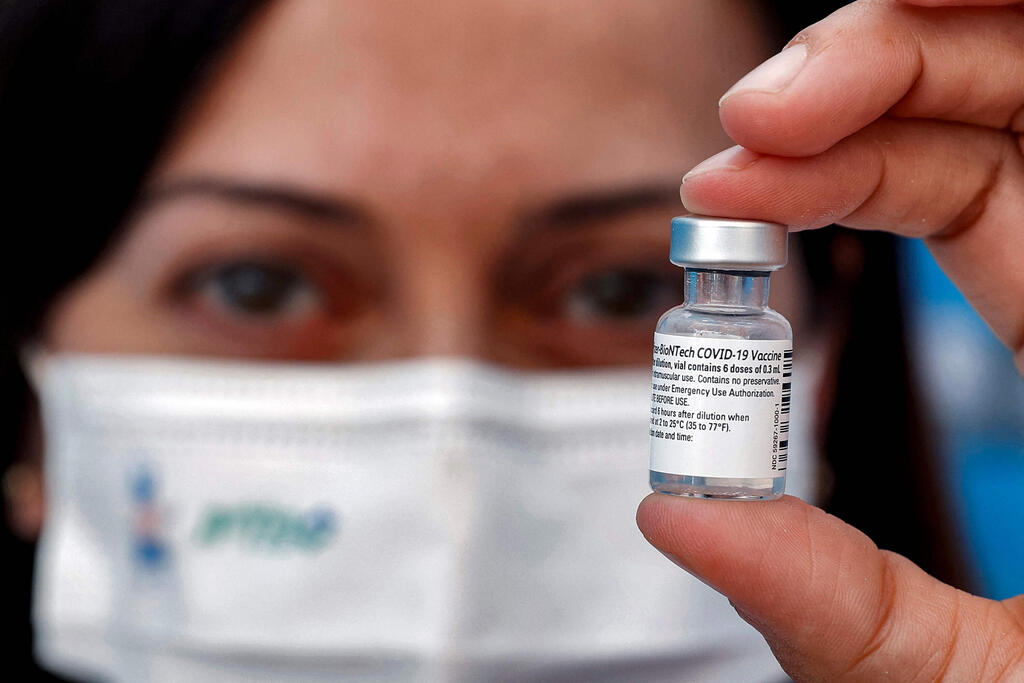

A medic holds up a vial of the Pfizer-BioNTech COVID-19 coronavirus vaccine at a vaccination center at the Atid al-Najah High School for the Sciences at the city of Taibeh in northern Israel on August 19, 2021
(Photo: AFP)
Rare cases of myocarditis, an inflammation of the heart muscle, have been reported in people who have received the Pfizer vaccine, especially in young men.
Separately, the EMA has given the green light to people with "severely weakened immune systems" to get additional doses of Moderna and Pfizer at least 28 days after their second dose.
Sometimes two doses are not enough to produce enough antibodies in people who are immunocompromised, such as people who have had an organ transplant.
"Although there is no direct evidence that the ability to produce antibodies in these patients protects against Covid-19, the additional dose is expected to increase protection at least in some patients," declared the EMA.

