Getting your Trinity Audio player ready...
Israel announced on Monday will offer the coronavirus vaccine booster three months after the two initial doses.
The new timeframe would be applied to vaccines made by Pfizer, Moderna and AstraZeneca.
3 View gallery

A medic handles a vial of Pfizer's coronavirus vaccine at a clinic in East Jerusalem
(Photo: AFP)
It was also announced that those who had recovered from the disease will be able to receive the booster after three months.
The Health Ministry said that due to the rapidly spreading Omicron coronavirus variant, "there is a rising need in boosting the population's protection [against the virus] as quickly as possible."
It also said that it was following similar steps by other countries such as the UK, Germany and France.
The Health Ministry earlier reported its highest single-day leap in coronavirus cases in months after 1,760 Israelis have tested positive for pathogen out of some 94,300 tests conducted the previous day but hospitalizations remained stable across the country.
Israeli hospitals were treating 87 COVID-19 patients in serious condition, down from 96 on Sunday. Health Ministry data showed that nearly 86% of severe cases were reported among unvaccinated patients. The unvaccinated also accounted for over 95% of all severe cases in the under 60 age group.
Surging morbidity is largely attributed to the rapid spread of the new Omicron variant throughout the country, cases of which are doubling every two and a half days according to Health Ministry data presented to the government Monday morning.
According to the findings, 50% of all virus carriers who went through an epidemiological interrogation were infected with the new strain.
As of Saturday evening, 1,118 Omicron cases were detected in Israel, 723 of which in travelers returning from abroad, but estimates place that figure much higher.
Sheba Medical Center in Ramat Gan began administering the fourth dose to a test group of 150 of its medical staff who have shown to have a low COVID-19 antibody count months after receiving the third shot in hopes of learning of the shot's efficacy.
3 View gallery
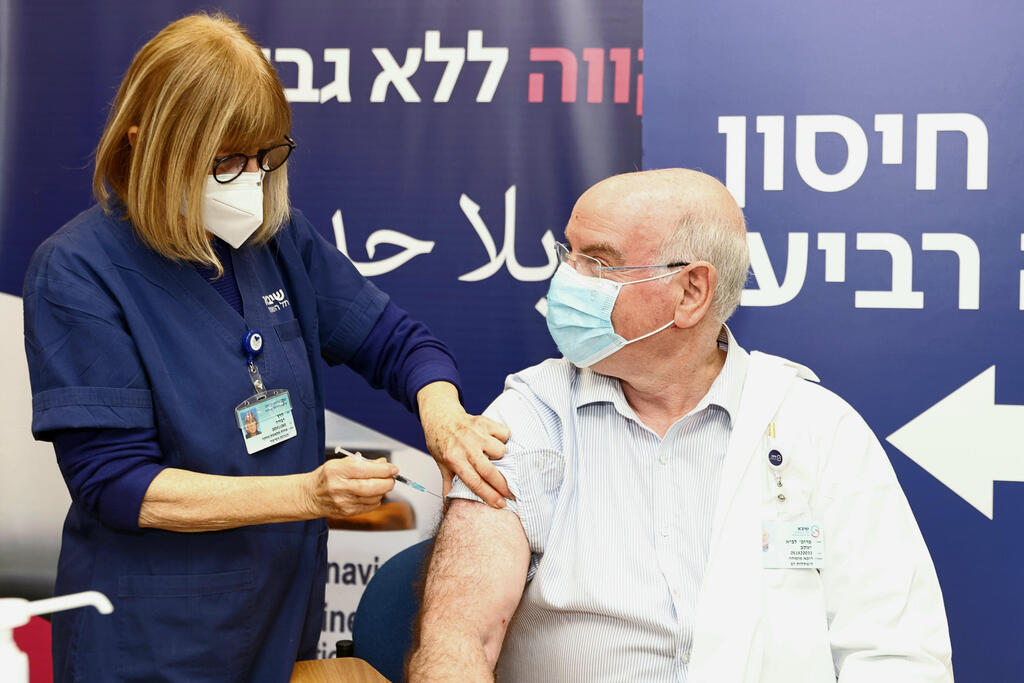

Sheba Medical Center's director of the Heart Transplantation Unit, Prof. Jacob (Jay) Lavee, receives his fourth dose of the coronavirus vaccine
(Photo: Avigail Uzi)
The hospital's director of the Heart Transplantation Unit, Prof. Jacob (Jay) Lavee, was the first subject to take part in the trial.
"As far as I know, I am the first healthy person in the world to receive the vaccine. I know of some immunosuppressed patients who have already received the vaccine in the United States. I feel great," Lavee told Ynet.
"I did not have any qualms about taking part in the trial for two reasons — first, out of the desire to protect myself, since as part of the previous trial on Sheba employees, I know that my immunity level has already dropped below the desired threshold after the third vaccine, but no less important is the will to avoid passing the illness to our patients, especially the heart patients I see in particular and all other patients here at Sheba. I think it's paramount that the medical teams won't make a vector for the disease to spread."


