Getting your Trinity Audio player ready...
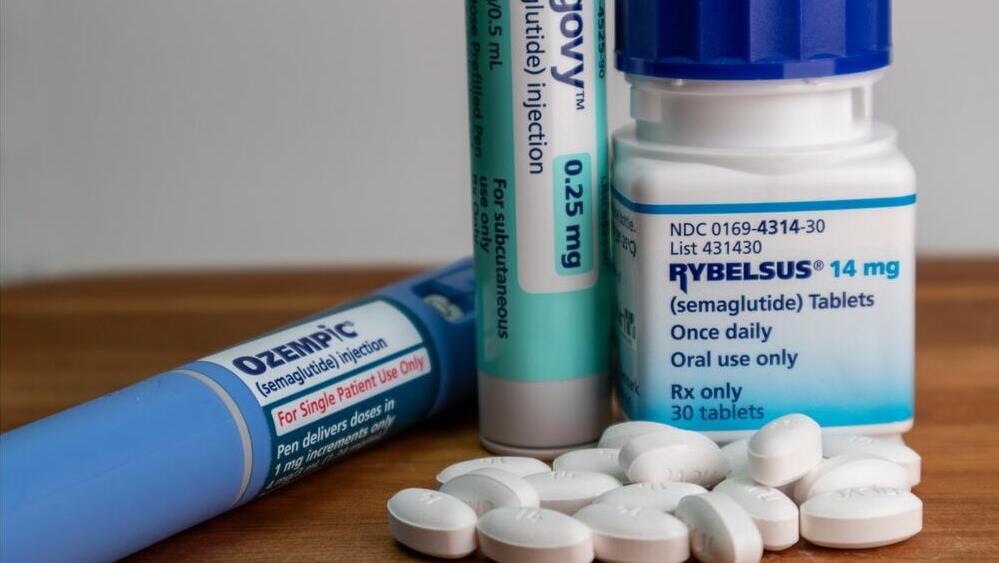
Rybelsus (oral Ozempic) reduces the risk of major cardiovascular events, including heart attacks and strokes, by 14% in people with type 2 diabetes, according to a study presented over the weekend at the annual American College of Cardiology (ACC) conference and published in the New England Journal of Medicine.
The drug, already included in Israel's health basket for all diabetes patients, is expected to bolster pharmaceutical companies' efforts to introduce more oral weight-loss medications in the coming years.
The U.S. Food and Drug Administration (FDA) approved oral semaglutide, marketed as Rybelsus by Novo Nordisk, six years ago for adults with type 2 diabetes. Two years ago, the FDA updated its label to allow first-line use.
The SOUL study, presented at the ACC conference in Chicago, is the first to examine the cardiovascular benefits of an oral glucagon-like peptide-1 (GLP-1) receptor agonist. The drug's active ingredient mimics the human GLP-1 hormone and binds to its receptors.
The study tracked high-risk type 2 diabetes patients with heart disease, chronic kidney disease or both for nearly five years. Researchers found a 14% reduction in major cardiovascular events, including cardiovascular death, heart attack or stroke, among those taking the drug. Nonfatal heart attacks dropped by 26%. No significant impact on kidney function was observed.
The findings position Rybelsus as a potential alternative to injectable diabetes treatments. While the mechanisms behind GLP-1’s cardiovascular benefits are still under investigation, researchers believe its anti-inflammatory properties play a key role.
“This study gives us confidence in prescribing an oral tablet for those hesitant to take injections,” said Dr. Darren K. McGuire, a professor of medicine at the University of Texas Medical Center and the study’s lead researcher. “Whether taken as a pill or an injection, these drugs rapidly reduce systemic inflammation.”
 Prof. Michael Shechter
Prof. Michael ShechterProfessor Michael Shechter, head of the clinical research unit at Sheba Medical Center, who led the SOUL study in Israel, stressed the significance of the findings. “A 14% reduction in major cardiovascular risk is substantial. For comparison, advanced cholesterol-lowering injections given biweekly reduce risk by 15%,” he said.
“For the first time, there’s a simple, accessible and safe oral treatment that addresses both diabetes and cardiovascular disease.”
<< Get the Ynetnews app on your smartphone: Google Play: https://bit.ly/4eJ37pE | Apple App Store: https://bit.ly/3ZL7iNv >>
The phase 3 study included 9,650 type 2 diabetes patients with coronary artery disease, stroke or chronic kidney disease. It began in 2019 and concluded in late 2024. Of the participants, 70% had coronary artery disease, 42% had chronic kidney disease, 21% had a history of stroke and 15.7% had peripheral vascular disease.
Additionally, one in four had heart failure. All patients received standard care, half given Rybelsus and half given a placebo.
“This is the first large-scale international study proving that oral semaglutide significantly reduces cardiovascular and cerebrovascular events in diabetes patients with heart disease or stroke, with or without kidney failure, while on optimal treatment,” Shechter said.
He added that Israel played a key role in the study, contributing 80 patients from eight medical centers. “One in three people with type 2 diabetes also has heart disease. It’s critical to provide treatments that address both conditions together,” he said.
Following the study’s positive results, Novo Nordisk announced plans to seek regulatory approval in the U.S. and the EU to expand Rybelsus' label for reducing cardiovascular risk in adults with type 2 diabetes.
The company is betting on these findings to strengthen its market position after its stock plummeted 25% in March — the steepest monthly drop since July 2002. According to Reuters, investors are concerned that the weight-loss drug pioneer is losing ground to U.S. rival Eli Lilly, whose shares have risen 6% this year. Despite these concerns, Novo Nordisk’s sales of Wegovy more than doubled in the fourth quarter of last year.